A number of manufacturers of lubricating eye ointments have been recalled for an an infection danger after federal inspectors discovered sterile situations on the Indian plant the place the merchandise have been manufactured.
The recall of Brassica Pharma Pvt. in Thane, a metropolis within the Indian state of Maharashtra, comes after a lethal epidemic final 12 months of eye infections linked to synthetic tears made by one other Indian firm.
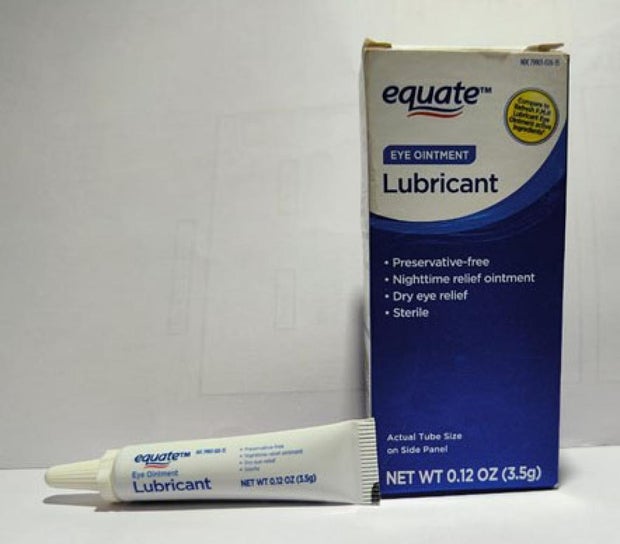
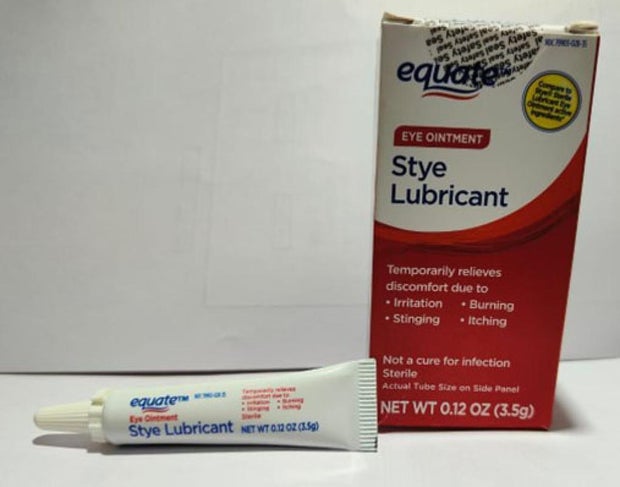
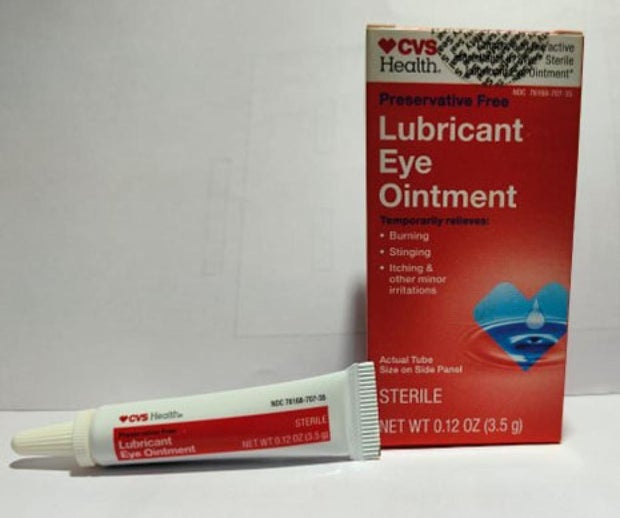
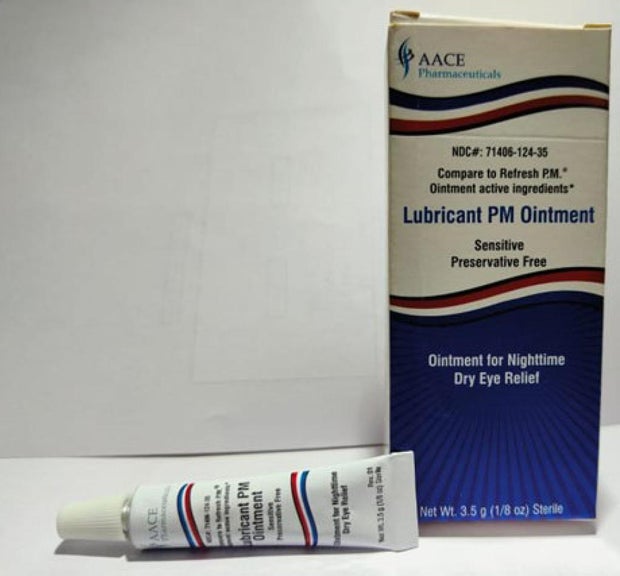
Offered nationwide by retailers together with CVS Well being and Walmart, the newest recall entails 4 merchandise beneath the manufacturers Equate, CVS Well being and AACE Prescription drugs, in line with the discover issued Monday by the Meals and Drug Administration of america.
All have an expiration date from April 2024 to September 2025. The recalled merchandise embody:
- Equate Lubricant Eye Ointment in a 3.5 gram tube, packed in a field with UPC code: 681131395298.
- Equate Model Lubricant Eye Ointment in a 3.5 gram tube in field with UPC code: 681131395304.
- CVS Well being Lubricant Eye Ointment in a boxed 3.5 gram tube with UPC code: 050428634141.
- Lubricant PM Ointment in a 3.5 gram tube, bought in a field with the UPC code: 371406124356.
US Meals and Drug Administration
US Meals and Drug Administration
US Meals and Drug Administration
US Meals and Drug Administration
Individuals ought to cease utilizing the recalled eye ointments and might return them to the place of buy. These with questions can name 1 833-225-9564 or information@brassicapharma.com.
EzriCare synthetic tears have been among the many manufacturers recalled final 12 months by Delsam Pharma of Chennai, India, as well being officers recognized 81 individuals in 18 states affected by infections, with 4 deaths and a number of other circumstances of sight loss reported.
In January, the FDA warned in opposition to it eye drops copy due to the chance of an infection.